OncoSilTM for Patients

About Pancreatic Cancer
Pancreatic cancer is a type of cancer that begins in the pancreas, an organ located behind the stomach. The pancreas plays a crucial role in digestion by producing enzymes that help break down food, as well as hormones, including insulin, that regulate blood sugar levels.
Pancreatic cancer typically develops when cells in the pancreas begin to grow out of control and form a tumour. There are two main types of pancreatic cancer:
Exocrine tumors: These are the most common type of pancreatic cancer, accounting for about 95% of cases. They start in the cells that produce enzymes for digestion and are called adenocarcinomas.
Endocrine tumors: Also known as pancreatic neuroendocrine tumors (NETs) or islet cell tumors, these are much less common and develop in the hormone-producing cells of the pancreas.
Pancreatic cancer is often difficult to detect in its early stages because it may not cause noticeable symptoms. As it progresses, symptoms may include abdominal or back pain, jaundice (yellowing of the skin and eyes), unexplained weight loss, loss of appetite, and digestive problems.
Risk factors for pancreatic cancer include smoking, obesity, diabetes, chronic pancreatitis (inflammation of the pancreas), family history of pancreatic cancer, and certain genetic syndromes.
Treatment options for pancreatic cancer depend on the stage of the cancer, its location, and the overall health of the patient. They may include surgery, chemotherapy, radiation therapy, targeted therapy, and immunotherapy.
OncoSil™ System
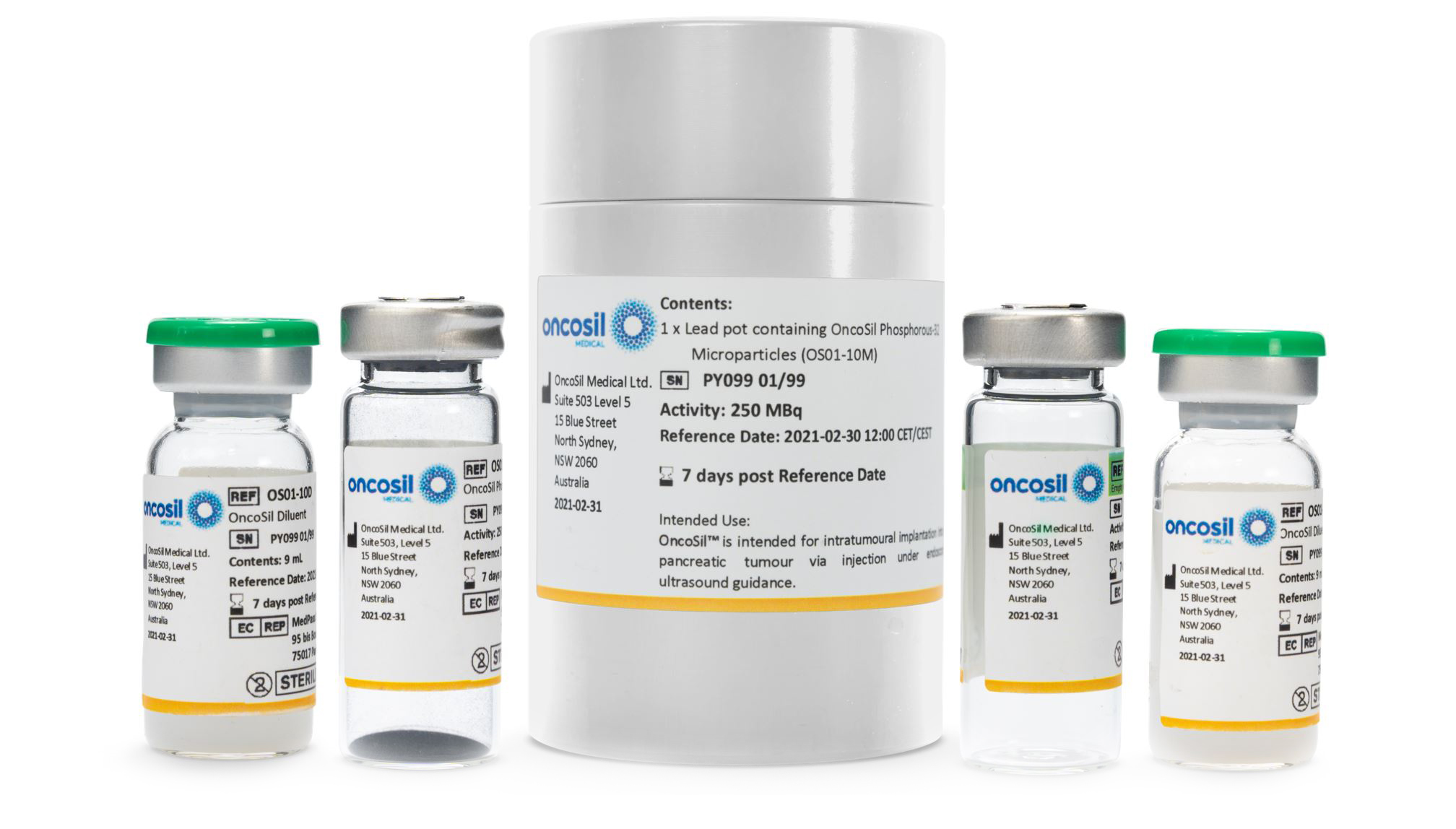
OncoSil™ is a single-use brachytherapy (internal radiation) device used to deliver a pre-determined dose of beta radiation directly into cancerous tissue.
OncoSil™ carries the active component radioactive Phosphorous (P-32) microparticles. These microparticles are tiny, and range in diameter from 28 to 32 micrometres, which is smaller than the width of a human hair. Once implanted, the OncoSil™ microparticles will remain permanently in your tumour.
OncoSil™ is injected directly into the pancreatic tumour under endoscopic ultrasound image guidance.
The purpose of the OncoSil™ device is to deliver radiation from Phosphorous-32 (P-32) directly into your tumour to destroy cancer cells. Once implanted, 98% of the radiation will be delivered in 81 days and the OncoSil™ microparticles will remain permanently in your tumour; they have been tested to ensure long-term safety.
The beta radiation emitted by the OncoSil™ travels a short distance to the tumour tissue causing direct damage to the cancer cell DNA. This damage makes the cancer cells incapable of further cell division and proliferation. By stopping cell division and proliferation, OncoSil™ can prevent the cancer cells from multiplying and may ultimately shrink the tumour mass as the cancer cells eventually die.
OncoSil™ Mode of Action
Frequently Asked Questions
Who performs the OncoSil™ treatment?
The OncoSil™ implantation procedure is performed in a licensed treatment facility by an endoscopist and a nuclear medicine physician.
Who is OncoSil™ suitable for?
OncoSil™ is intended for the treatment of patients with locally advanced unresectable pancreatic cancer, in combination with gemcitabine-based chemotherapy.
Will I have to stop my chemotherapy to receive OncoSil™?
Generally, the OncoSil™ treatment is performed in the resting week of chemotherapy cycles, so there is no need to interrupt the chemotherapy. However, chemotherapy should not be administered within 48 hours either side of OncoSil™ implantation.
Should I stay at the hospital after the OncoSil™ treatment?
OncoSil™ implantation is an outpatient procedure, although some patients may be required to stay overnight depending on the treating clinician’s assessment or local regulations.
What are the possible side effects related to the implantation procedure?
Like other endoscopy procedures, endoscopic ultrasound is typically well tolerated. However, potential risks include:
- Pain, fever, feeling sick and dizzy or faint, bloating and/or a sore throat, however these symptoms are usually short-lived and easily treated
- Side effects related to medicines used as part of the procedure such as the sedative or anaesthetic
- Incorrect placement of OncoSil™ Microparticles
- Uncommon or rare complications of an endoscopic procedure include bleeding or a tear of the stomach or duodenum. In addition, with needle insertion into the pancreas, there is a small risk of bleeding, infection or inflammation (known as pancreatitis)
- Following treatment with OncoSil™, some radioactivity may be detected in faeces (stools) or in the blood and urine. These amounts are small and have not been associated with any harm
What are the possible side effects related to the OncoSilTM treatment?
In a clinical trial looking at the safety and efficacy of OncoSil™, the majority of side effects were believed to be due to either chemotherapy or cancer. However, those side effects that were possibly or probably related to OncoSil™ treatment and/or the endoscopy procedure included:
- Fatigue or tiredness
- Abdominal pain or discomfort
- Gastrointestinal effects – in particular, nausea, indigestion and reflux
- Abnormal blood results – in particular, reduced white blood cells and platelets in the blood
What are the possible side effects related to interactions with other medicines you are taking at the same time?
The safety of OncoSil™ has not been established in the presence of other medicines, with the exception of certain chemotherapy drugs (gemcitabine + nab-paclitaxel). If you are taking other medicines at the same time you are to receive OncoSil™, you should discuss this with your doctor.
What are the radiation safety considerations following the OncoSil™ implantation?
The radiation will only travel a short distance (i.e. 2.76 mm on average) inside your tumour and therefore very little radiation will leave your body. Whilst this does not represent a radiation hazard to your family or members of the public, there may be a small amount of radiation present in your urine, blood and/or faeces, and therefore you should follow some important safety practices. Follow this guidance for two weeks after your OncoSil™ treatment:
General interactions
- Groups vulnerable to radiation exposure, such as pregnant women, infants and children, should avoid unnecessary contact with the patient for two weeks.
Bathroom use
- Flush the toilet twice after use Wipe toilet seat and handle with a disinfecting wipe (Clorox or similar) folding it inside itself, so that used sections are covered
- Wash hands thoroughly with soap and warm water
General clean up
- If any spills of bodily fluids occur, promptly clean up, wearing disposable gloves
- Place gloves and all articles used in the clean-up in a bag and dispose of them in normal household waste
- If any bodily fluids transfer to clothing or bottom of shoes, promptly wash them separately
What if I am pregrant or breastfeeding?
The safety of OncoSil™ has not been established in patients who are pregnant or who, within 12 months of implantation, become pregnant, nor has the safety of OncoSil™ been established for future children of patients who are pregnant at the time of implantation, or who, within 12 months of implantation, become pregnant. Therefore, the use of contraceptives by both female and male patients is recommended for a period of 12 months following the OncoSil™ treatment.
The safety of OncoSil™ has not been established in children being breastfed by patients at the time of implantation or subsequent to implantation. Therefore, it is recommended that patients do not breastfeed for a period of at least 12 months following an OncoSil™ treatment.
Resources

A Patient’s Guide to OncoSil (English)

A Patient’s Guide to OncoSil (German)

A Patient’s Guide to OncoSil (Italian)

A Patient’s Guide to OncoSil (Spanish)

A Patient’s Guide to OncoSil (Turkish)

A Patient’s Guide to OncoSil (Hebrew)
Treatment Centres
DISCLAIMER: The information provided on this site is compiled by OncoSil Medical for reference purposes only, assisting in the location of physicians who have used or are using OncoSil. This database may not include all physicians that use or have used OncoSil, and therefore should not be considered exhaustive or definitive.OncoSil Medical does not, under any circumstances, verify, monitor, or endorse the license, credentials, accreditations, qualifications, or the quality of care of any physician listed in this database. OncoSil Medical expressly disclaims any responsibility or liability for any treatment or other medical advice provided by any physician included in this database.
Users of this database should not rely solely on the information provided here for choosing a physician. It is recommended that users make their own independent inquiries and seek the advice of a qualified healthcare professional before choosing their healthcare provider.













